Quote: billryanhttps://news.yahoo.com/will-elizabeth-holmess-conviction-change-silicon-valley-223806861.html
Eddie Lambert legally raped Sears. The BOD at CYDY is raping the company and its shareholders. The wheels of Justice turn slowly but this current Justice Department is slowly reintroducing the rule of law and the Holmes' conviction may mark the end of "the fake it until you make it" era of putting out misleading press releases that scream one thing but hide the truth on page 27.
If you want to get rich with pink sheet stocks, get a job selling them.
link to original post
Cue George Costanza asking, "Was that wrong?"
I definitely agree with you on Lampert and should probably see what ended up happening (if anything) with that, but I think he has enough of an argument that it won't rise to the level of anything criminal, in his case. He maintains that he took huge personal losses in his running of the company (making loans to the company, etc) and I tend to believe that. I guess you can make an argument, and it would be valid, that it's not a legitimate way to try to recoup anything and wasn't done with the best interests of SHLD at heart, but again, he can fall back on SHLD needed the short-term cash infusions more immediately than having open bidding on the real estate in question was going to be able to accomplish.
In any event, SHLD was screwed from the word go---and some of that predated Lampert. Here are the major problems, as I see them:
1.) Increased competition from superstores such as Walmart and Target. (This would be even more detrimental to KMart than Sears operations)
2.) Inability to modernize the stores. Basically, they couldn't even really do anything to clean up the stores, much less do full across-the-board renovations to bring them out of the 1980's/early 1990's. Walmarts are obviously not the nicest places in the world, nor are they designed to be, but your average Walmart was only several years old and positively glamorous compared to your average KMart location.
3.) The customer service at KMart and Sears was dreadful and the stores were run by skeleton crews. Again, the stores were mostly operating at losses as it was, (forget about the debt that the company had---the stores were mostly operating at a loss anyway) so it's kind of a hard sell to tell them they should be spending more money to better serve the customers that they barely have to begin with.
4.) The websites were dreadful.
5.) Amazon. (And other online retailers)
6.) Inability to, "Right size," their retail footprint. That's just a fancy way of saying that all of the stores were too big and they had no way of getting into smaller (and cheaper to operate) retail spaces because they either owned or partially owned the real property, or alternatively, were locked into long-term leases. With that, you either end up stocking a bunch of excess inventory that you basically have no hope of ever selling, or alternatively, a ton of empty floor space.
7.) Inability to, "Right-size," their distribution channel. Basically, their entire distribution network only made sense if you have a place with thousands of stores. Naturally, you can shutdown some distribution centers, but then you're still paying a ton of money on transporting goods to the stores that are further away from what distribution centers do remain.
So, what could Lampert have done? I don't think anything. I think Sears and KMart were both going to be massively screwed no matter what.
I guess the main reason for the Sears/Kmart marriage (2004/2005) was cost savings on distribution channels, but that's only going to do so much. You really can't even run Sears/KMart as one thing because they had totally different customer demos---so really nothing about it made sense.
I don't know. I think there would only be anything worth pursuing if Lampert somehow managed to be personally enriched in these actions in that nearly 15 year period, but I tend to think that he personally also lost massive amounts of money.
Quote: mcallister3200Öother than the dopamine rush of getting something new.
link to original post
Most people would like to rev up their dopamine receptors without the hazards of a gambling addiction or meth.
They failed to adapt to the changing retail environment and Lampert took advantage of it. I hate what he did, but I don't think it was criminal, in a legal sense. What is going on at CYDY is a whole other matter.
Lampert can claim to have lost hundreds of millions, but I think he is much richer today that when he started.
Below are facts. Most come from the Cytodyn vs. Amarex injunction lawsuit. Lawsuits can be viewed on Pacer.com
2014 Cytodyn inks an MSA (Master Services agreement) with Amarex.
Amarex is a 3rd party vendor specializing in clinical trials. They collect trial data, organize and per the MSA, they were to handle FDA submissions when required.
(A 3rd party vendor is required for data collection as having an applicant do it would be conflict of interest.)
Kazem Kazempour is the CEO of Amarex. He has 35 years experience in clinical trials including five years AS AN FDA REVIEWER (Remember this it's important)
From 2014 - 2020 Cytodyn pays Amarex $80 million dollars for their services.
2014 - 2018 clinical trials for Leronlimab for HIV involving over 800 patients occur. Many of them are still receiving Leronlimab now after 7 years.
2018 Leronlimab MEETS ENDPOINTS on Phase 3, placebo controlled, double blind study for HIV in combination with HAART therapy. (Leronlimab is now statistically proven to work for the above indication)
The next phase is to apply for a license to sell Leronlimab from the FDA (BLA or Bliologics Licence Application)
A BLA is MOUNTAINS of clinical data culled from all the trial sites in the relevant trial Average time to prepare a BLA is one year. Average time for FDA to analyze and then approve is another year.
2018 Amarex begins the year long process of preparing the BLA. This isn't done without FDA oversight. An FDA reviewer is assigned and deals directly with the company. The company applicant is of course Cytodyn but per the MSA, Amarex is handling the prep. The FDA reviewer is dealing directly with Kazem Kazempour of Amarex who to reiterate is a former FDA reviewer himself.
April 2020 After two years Amarex has still not completed the (normally one year) preparation of the BLA.
In a controversial email CEO of Cytodyn Nader Pourhassen is told Amarex needs one week. Pourhassen angrily says to just submit the BLA and whatever deficiencies need to be filled in later they will deal with.
April 27, 2020. The BLA is filed with the FDA.
April 30, 2020 Nader Pourhassen received a milestone payment for the filing of the BLA (stock options which he sells a portion of)
June 2020, The FDA begins informing Cytodyn that things don't look good. The BLA is a mess. Also, the FDA has uncovered that Amarex has backdated documents. This means they visited trial sites and changed the dates on documents to cover up deficiencies in their data collection.
Remember that some patients from the HIV trials are still receiving Leronlimab. Data collection is still ongoing! Amarex continues to bill Cytodyn but Cytodyn now stops payment due to the FDA notes.
July 13, 2020. The FDA issues a RTF (Refuse To File).
RTF is not a rejection of the drug. It's a refusal of the data. The BLA takes the FDA a year to review and they charge millions of dollars. Prior to the review as a courtesy, the FDA does a preliminary. In this case they warned that based on a preliminary review too much data was missing to pass the review. Instead of wasting FDA resources and being billed millions, the RTF listed the deficiencies and the HIV BLA was not filed. It's now up to the company to fix the BLA.
The RTF is 20 pages long. Here are some of the most egregious issues.
1) Kazem Kazempour of Amarex had a discussion with the FDA reviewer in 2019. A protocol was determined. Nonetheless Kazempour of Amarex (a former FDA reviewer himself) changed the protocol with no explanation.
2) over the course of 18 months the FDA reviewer asks Kazempour to supply RO Assays. After a year and a half, the reviewer scolds Kazempour for ignoring his repeated requests.
3) Thousands of fields of data are missing. The FDA reviewer notes the data is in their possession because they are in.xpt files. Kazempour needs to complete his work.
4) inexplicably with thousands of fields missing data, the FDA reviewer asks why Kazempour filled in fields meant to be left blank.
5) Data from different trials is mixed up. The FDA reviewer suggests Amarex has faulty data collection.
6) patient discrepancy is noted by the FDA reviewer as he again calls into question Amarex collection methods.
In particular one patient is reported deceased. But one month later this same patient is complaining about a right arm abscess.
After receiving the RTF, Cytodyn demands an audit of Amarex. Amarex refuses. It is contractually required in the MSA that Amarex must allow an audit. It's also an FDA regulation.
Cytodyn demands their database information (EDC) and master trial files be handed over. Amarex refuses. Again this is in violation of both the MSA contact and FDA regulations.
Amarex cites the unpaid bill as their reason for not handing over data or allowing an audit.
Cytodyn Sue's for arbitration in the matter of the bill and an injunction for the data.
December 2021, the judge awards the injunction.
Amarex must hand over the EDC immediately
Amarex must finish handing over the Master trial files by February 1st, 2022
Amarex must submit to an Audit to be completed by February 11, 2022.
And that's where we are at. The BLA appears to have been scuttled by Kazem Kazempour of Amarex. The backdated documents appear to show that it was intentional.
The FDA has said Amarex collection appears flawed.
Cytodyn must now analyze the data currently being handed over so they can resubmit for the BLA (that puts FDA approval out to next year (2023) at the earliest.
The audit (by a 3rd party auditor) will analyze what went wrong with Amarex. Answers forthcoming.
Lots of conspiracy theories about someone compromising Amarex and Kazempour but I don't wish to speculate.
Quote: billryan
No, can you please teach me how the stock market works. I've evidently been doing in all wrong since I bought my first share of Sears in 1967.
Evidently I've been playing BJ wrong as well. Who knew you were supposed to wager your entire bankroll on the first bet.
link to original post
Sadly, Sears is at $0.03 a share so Cytodyn wins since it as at $0.70 a share
April 27, 2020. The BLA is filed with the FDA.
April 30, 2020 Nader Pourhassen received a milestone payment for the filing of the BLA (stock options which he sells a portion of)
That is the problem, in a nutshell. Nader ordered a report he knew to be no good to be filed so he could cash in on a bonus he should not have collected.
No third party screwed the company. The company's CEO screwed the stockholders, again.
Quote: DRichQuote: billryan
No, can you please teach me how the stock market works. I've evidently been doing in all wrong since I bought my first share of Sears in 1967.
Evidently I've been playing BJ wrong as well. Who knew you were supposed to wager your entire bankroll on the first bet.
link to original post
Sadly, Sears is at $0.03 a share so Cytodyn wins since it as at $0.70 a share
link to original post
I did pretty well with my Sears stocks. Once I started earning some money, I reinvested the dividends, and they grew for about twenty years. I sold a chunk of it in 1998 when I made a big purchase and sold the rest around 2002. I did buy some around 2008 and sold that for a pretty big loss but it was only a few dozen shares.
Quote: billryanIn a controversial email CEO of Cytodyn Nader Pourhassen is told Amarex needs one week. Pourhassen angrily says to just submit the BLA and whatever deficiencies need to be filled in later they will deal with.
April 27, 2020. The BLA is filed with the FDA.
April 30, 2020 Nader Pourhassen received a milestone payment for the filing of the BLA (stock options which he sells a portion of)
That is the problem, in a nutshell. Nader ordered a report he knew to be no good to be filed so he could cash in on a bonus he should not have collected.
No third party screwed the company. The company's CEO screwed the stockholders, again.
link to original post
Unfortunately the judge didn't agree with you.
This very subject was brought up in court.
Guess what. Bill Ryan knows better than federal judges now. Good luck with that.

Some people think the two companies have similar MO. In other words Cytodyn is a fraud just like Theranos.
Theranos promoted a product that was fake. It didn't even exist and to make it's investors believe it does exist they created faked scenarios.
Theranos used regular equipment to show results they claimed their equipment did, they actually built a temporary fake laboratory to show off to visitors.
Now I know some idiots think Leronlimab is a faked molecule or snake oil. In other words, it has no chance of ever working as a treatment for disease because it is a con job.
In a post above I showed how Amarex screwed up Cytodyn by seemingly purposefully doing a reckless job. That reckless job resulted in the FDA issuing a twenty page RTF, (Refuse To File) filled with all the reasons why
It's a rebuke from the FDA but I want you to read just one section. It's filled with medical jargon but please read it even if you don't understand. I chose this one because the FDA reviewer is trying to understand why the worker from Amarex (a former FDA reviewer himself) changed the agreed upon protocols.
So here it is and I will comment on the other side.
"Submission of Unagreed Upon Population PK (Pop PK) Analysis: A thorough assessment of dose selection is critical to ensure the safe and effective use of drugs. Methodologies such as Pop PK analysis are used to assess dose selection and to evaluate the impact of intrinsic and extrinsic factors on drug exposure. Hence, it is critical that the data used to build and validate population pharmacokinetic models are robust. Therefore, we requested a Pop PK analysis to support the selection of a higher dose [700 mg, based on the dose-finding study in the monotherapy study (CD03)] than the dose evaluated in the pivotal trial (CD02), because you only analyzed the PK data at the proposed dose (700 mg) using Pop PK analysis method. In your correspondence dated January 16, 2019, you described plans to use studies CD02, CD03, and CD06 studies for the Pop PK model development and covariate analysis. Rather than submitting a Pop PK analysis as described in that correspondence, the submitted analysis in your BLA includes data from CD02, CD03, and Study 2101 without a clear rationale for including the results from study 2101 and excluding the results from trial CD06. Of note, the formulation used in Study 2101 (formulation two) is significantly different from the formulation used in CD02, CD03, and CD06 (formulation four) and the current PK sampling times across the formulations do not support a comparative test via Pop PK. In the absence of comparability data between formulations two and four, a substantive review of the Pop PK analysis to assess the proposed dosing regimen cannot be conducted."
Okay whether Leronlimab is shown to work (it ALREADY HAS FOR HIV which is why they are applying for a license to sell it) the above quote tells us one thing.
There is 0.000000 chance that an FDA reviewer has spent years doing that type of analysis on the Leronlimab molecule and has been fooled by a "fake" drug.
The drug has been administered to over a thousand patients with all types of biomarkers collected (tens of thousands of data points) over a decade. Medical professionals including the FDA are examining this for nearly a decade, AND IT SHOWED STATISTICAL SIGNIFICANCE as examined by the FDA for treatment of HIV
And some idiot thinks that's a faked product like a jimmied up blood test machine from Theranos?
Come on, don't be an idiot.
Quote: GundyTheranos and Cytodyn have something in common- they are both worth $0.
link to original post
Not yet, Cytodyn is still worth $478 million. Probably not for long though.
This is a perfect example of why children shouldn't play with matches, or run with scissors.
Quote: DRichQuote: GundyTheranos and Cytodyn have something in common- they are both worth $0.
link to original post
Not yet, Cytodyn is still worth $478 million. Probably not for long though.
link to original post
Earnings week.
Quote: billryanhttps://news.yahoo.com/will-elizabeth-holmess-conviction-change-silicon-valley-223806861.html
Eddie Lambert legally raped Sears. The BOD at CYDY is raping the company and its shareholders. The wheels of Justice turn slowly but this current Justice Department is slowly reintroducing the rule of law and the Holmes' conviction may mark the end of "the fake it until you make it" era of putting out misleading press releases that scream one thing but hide the truth on page 27.
If you want to get rich with pink sheet stocks, get a job selling them.
link to original post
Quote: billryanI just looked over the thread and the only person that is comparing the two companies seems to be dark oz. I don't think anyone should be called an idiot, but if he wishes to refer to himself as one, I guess we shouldn't disagree.
This is a perfect example of why children shouldn't play with matches, or run with scissors.
link to original post
Quote: billryanReading comprehension doesn't appear to be one of your strong suits. My post specifically refers to people writing misleading press releases, something cydy is being sued for. Only in America can someone make ridiculous claims about something going to end covid , and almost two years and 750,000 deaths later still insist they were right.
link to original post
So the press release that Elizabeth Holmes was convicted for fraud with Theranos was a misleading press release?
That's why you linked to it?
Let's talk after this weeks earnings come out.
Quote: DRichMy question to BillRyan and Darkoz is do you think CYDY stock will be above or below $0.77 one week from now (January 17th at close of market)? As a passive investor that is all I care about.
link to original post[/q
In a week, I'd guess down, but where anything is in a week is a crapshoot. In a year, I'd say almost certainly down. There is nothing there. It's really just a matter of how long the BOD will be allowed to borrow money to pay their ridiculous salaries. Let's see what the earnings say.The older I get, the better I recall things that never happened
Quote: DRichMy question to BillRyan and Darkoz is do you think CYDY stock will be above or below $0.77 one week from now (January 17th at close of market)? As a passive investor that is all I care about.
link to original post
Cytodyn was at $.35 before Covid and it's reliant now on getting the HIV BLA resubmitted and passed by the FDA.
So unfortunately I expect the price to possibly, perhaps likely, return to that level.
There is no revenue because this is a pre-revenue biotech so why Bill would want to see the earning report makes no sense to me. (There is expected some minor revenue from the Philippines which used it for Covid there. It's an unapproved drug at this point. What earnings?)
This is normal for biotech companies in drug development. It can take a decade. Unfortunately that's also why a large amount fail due to needed revenue sources while they work on development.
The drug has passed stat sig on HIV. They would not be allowed to file a license to sell Leronlimab to the FDA (the BLA or Biologics License Application) if the data wasn't proven to work on HIV.
It was always my fallback if they didn't pass the Covid trials when I invested. They would just follow through on HIV.
Trials are trials. They aren't guaranteed. Anyone who doesn't understand that should not be investing in biotech.
But the HIV trial has already concluded. Leronlimab works for HIV.
When I see a steaming pile of turds, my first thought isn't how can I make money off of it, it is to avoid it.
Quote: billryanI don't short sell. Never have, never will. I make my money the old fashioned way. I don't speculate.
When I see a steaming pile of turds, my first thought isn't how can I make money off of it, it is to avoid it.
link to original post
If you don't speculate then you don't invest in the stock market unless you are claiming some clairvoyance or insider trading scenario.
Why are you so interested in stocks then?
(Hint: comic books are highly speculative as an investment)
Quote: darkozQuote: billryanI don't short sell. Never have, never will. I make my money the old fashioned way. I don't speculate.
When I see a steaming pile of turds, my first thought isn't how can I make money off of it, it is to avoid it.
link to original post
If you don't speculate then you don't invest in the stock market unless you are claiming some clairvoyance or insider trading scenario.
Why are you so interested in stocks then?
(Hint: comic books are highly speculative as an investment)
link to original post
Reading comprehension doesn't appear to be one of your strong suits.
CYDY tripled in price between Thanksgiving 2019 and New Years 2020, starting the New Year well over a dollar and doubling shortly after. Pre-Covid. By the time anyone knew of Covid, the price was well above what it is today.
Quote: billryanDO says cydy was thirty five cents before covid, which is like me saying I was 150 pounds. In Eighth Grade.
CYDY tripled in price between Thanksgiving 2019 and New Years 2020, starting the New Year well over a dollar and doubling shortly after. Pre-Covid. By the time anyone knew of Covid, the price was well above what it is today.
link to original post
Yes, that's correct. Shortly before Covid the price had a bump.
The HIV BLA was getting underway so I stand corrected. Look at the big brain on Bill.
Quote: billryanYou get caught in another mistruth and rather than apologize for it, you resort to insults. If you didn't feel the need to make stuff up, we might just get along better.
link to original post
I resort to insults?
From the guy who says I have reading comprehension problems twice this morning
And what mistruth. November 2019 is most certainly before Covid like I said.
(Old news I know, but now it's firmly below a dollar.)
Quote: MDawgTo quote Henry James (Washington Square), The thing has happened! CYDY is below a dollar.
(Old news I know, but now it's firmly below a dollar.
link to original post
Well, that I can't dispute lol.
at 55 cents!
The drug works. Lives can be saved.
This is very sad. I feel like my faith in science and humanity and logic is being tested.
Read this article. It helps me batten down the hatches.
https://www.griproom.com/fun/why-your-stock-is-always-red?fbclid=IwAR0EXlS4CZzQe8Tg1liAEExfOw1qTysT0OQMJa7gVt2DqNVh4PtHk7JtqAI
61% short volume.
Sharks in the water.
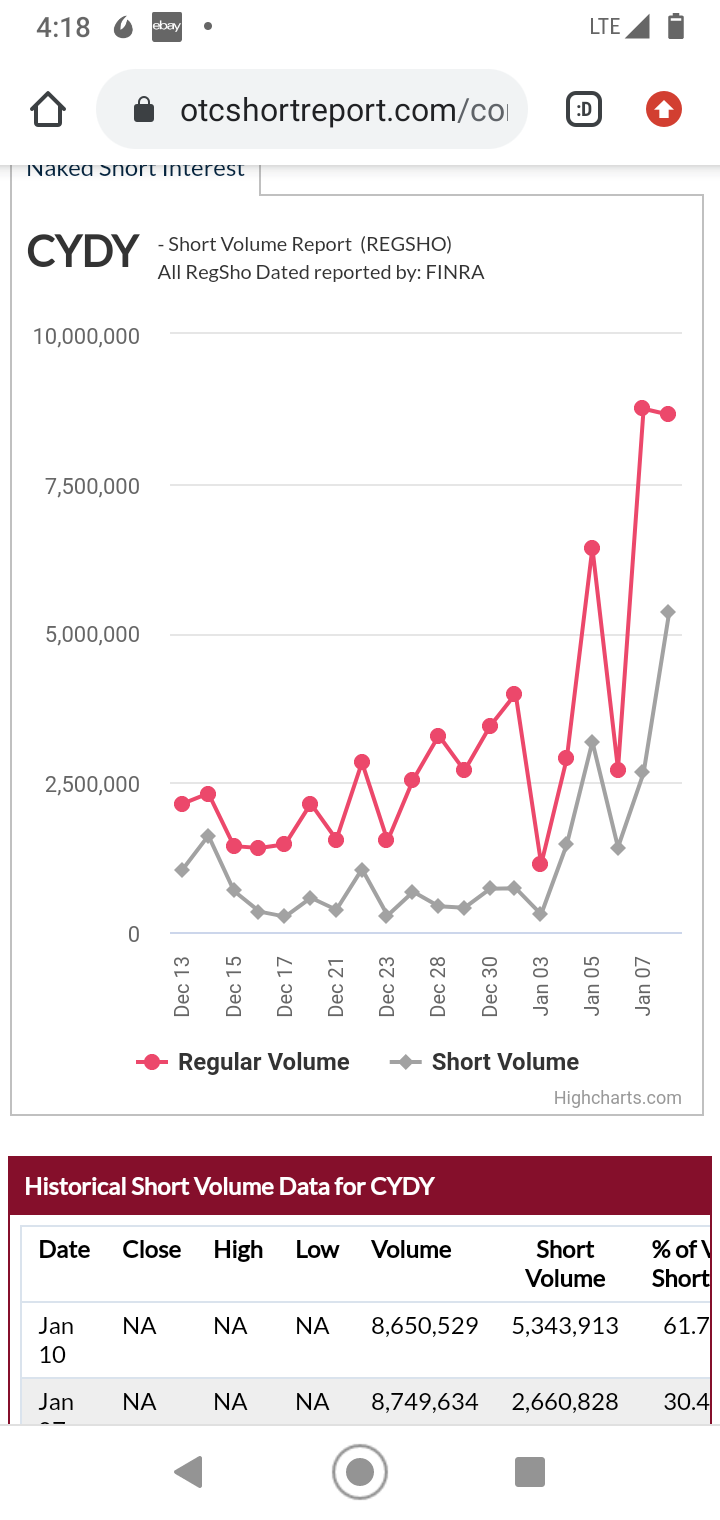
Quote: darkozShorts are attacking.
61% short volume.
Sharks in the water.
link to original post
I havenít checked recently, but Iíll bet Iíve been getting fifty cents a day loaning out my shares. Now that I rethink it, with the precipitous drop in price Iím probably getting a quarter a day.
It is now 12 or 13 days since the last investor's conference call was postponed. No news when it might be rescheduled.
CYDY has to post a huge cash bond in the next ten days for a trial to proceed.
I wouldn't be surprised if the stock gets a bit of a bump as the munchkins celebrate the house falling from the sky.
Quote: billryanCEO is gone. Ousted. I expected him to be perp walked, but that will come later.
It is now 12 or 13 days since the last investor's conference call was postponed. No news when it might be rescheduled.
CYDY has to post a huge cash bond in the next ten days for a trial to proceed.
I wouldn't be surprised if the stock gets a bit of a bump as the munchkins celebrate the house falling from the sky.
link to original post
I've been making up to $0.50 a day with my shares being lent out. I think now that the price has tanked probably only $0.20 a day. If new CEO has a good reputation (unlikely) then perhaps could get a bump.
Quote: billryanCEO is gone. Ousted. I expected him to be perp walked, but that will come later.
It is now 12 or 13 days since the last investor's conference call was postponed. No news when it might be rescheduled.
CYDY has to post a huge cash bond in the next ten days for a trial to proceed.
I wouldn't be surprised if the stock gets a bit of a bump as the munchkins celebrate the house falling from the sky.
link to original post
There is no trial. That's over.
The investigation (audit and data) that will show third party Amarex scuttled the HIV BLA requires the bond.
The science shows the drug works. I have always said I invested for the science. I certainly didn't invest for the CEO.
Really not sure what your point is.
What exactly is it you believe about Leronlimab (not lemonlabob, no idea what that is and perhaps why you are researching the wrong drug) because your claims have been all over the map. Like you have to keep pivoting your argument.
You seem to believe that the drug is worthless. You have called it snake oil.
Leronlimab has proven to work against HIV. Stat sig P-value proven. An HIV BLA was submitted to the FDA. You aren't allowed to even submit a license to sell the drug unless you have ALREADY proven both safety and efficacy in trials. If you say that's not true you are lying!
You don't like the CEO. You feel he is scamming (yet to be proven, no charges filed yet but regardless, he is if true scamming with a drug that actually works
I am invested in the science and the drugs ability to cure disease. It's to be seen which further disease it will be shown to work but it works on HIV.
Again, please don't show yourself to be a liar. Please don't say Leronlimab doesn't work
You should be celebrating, but it seems like you are incapable of spotting a rare win.
Quote: billryanYou just keep repeating the same nonsense over and over. If you want to attack my position, at least go back and try to understand what it is.
You should be celebrating, but it seems like you are incapable of spotting a rare win.
link to original post
You seem.unable to answer a simple question.
Do you feel Leronlimab works?
I remind you it has been proven to work against HIV.
What is your position on the subject?
If you hurry, you can still get in at the bargain-basement prices.
Buy as many shares as you presently own and you can cut your PPS in half.
Quote: billryanUp 15% for the day. And there was much rejoicing in the land.
If you hurry, you can still get in at the bargain-basement prices.
Buy as many shares as you presently own and you can cut your PPS in half.
link to original post
Yes I know how to buy shares.
Everyone makes mistakes.
You claimed Leronlimab was snake oil. I understand you decided this with less than one hour research.
You made a mistake. You didn't realize they had already passed a stat sig P-value trial proving it works.
You didn't want to believe or just didn't want to do the work to research (even though you put a lot of research into the stock price.)
People will respect someone who admits they were wrong. We are all human
I have clearly been wrong so far on where the stock would go as of now. I'm hoping that will change.
Now admit you were wrong when you claimed erroneously that the drug is useless.
Go ahead, show us who you really are. Own up to your error.
Seriously, why do you care if I think lemomlabob works or not?
You came on here telling everyone it was going to stop the covid pandemic well over a year ago. Obviously, it didn't. You thought trump was going to issue an emergency use order and he didn't
Now you seem to be claiming it works on HIV, yet it isn't approved for treating HIV after years of study. CYDY has failed to get FDA approval for just about everything. At this point, it looks like they are throwing it all against a wall and hoping something works. Just as they have been doing for almost a decade.
The person who was raping our company is gone. You should be happy, not worried if a person on a gambling website thinks lemonlabob is going to save the world for musical comedy.
Quote: billryanIs this another mouse vs rats moment for you?
Seriously, why do you care if I think lemomlabob works or not?
You came on here telling everyone it was going to stop the covid pandemic well over a year ago. Obviously, it didn't. You thought trump was going to issue an emergency use order and he didn't
Now you seem to be claiming it works on HIV, yet it isn't approved for treating HIV after years of study. CYDY has failed to get FDA approval for just about everything. At this point, it looks like they are throwing it all against a wall and hoping something works. Just as they have been doing for almost a decade.
The person who was raping our company is gone. You should be happy, not worried if a person on a gambling website thinks lemonlabob is going to save the world for musical comedy.
link to original post
So you just don't understand the FDA approval process.
I understand. It's difficult learning new things.
You ask why I care what you think but I ask why you care about my investment.
Quote: MDawgThere isn't yet any definitive proof that leronlimab works, is there? Not for HIV not for coronavirus. Jury is still out on all that.
link to original post
Wrong!
Sorry to burst your bubble.
It's funny how people just refuse to understand the approval process.
First the drug is trialed.
If it passes trials then it has been proven to work (in this case it was proven scientifically to work in HIV)
Once it is scientifically proven to work, the company files for a license to sell it on the market. FDA will not accept a license application without having first passed trials that show it works because what would be the point?
FDA accepted submission of the license BLA.
There was some data kerfuffle (missing data and a bunch of suspect entries) that the 3rd party company bungled and the BLA received notice Cytodyn had to fix the deficiencies.
Not deficiencies that prove the drug works or not but missing data (and we are talking a lot of data so not a simple fix) to resubmit the application.
They only need to resubmit. Why? Because the drug has already been proven to statistically work in HIV. They are seeking the license at this point.
To keep saying it hasn't been proven to work is just FUD.
Quote: MDawgThere isn't yet any definitive proof that leronlimab works, is there? Not for HIV not for coronavirus. Jury is still out on all that.
link to original post
Are you trying to bring facts into a rhetorical argument?


