Quote: darkozIt's absolutely hilarious that Bill can't seem to understand the difference between
"There is an investigation"
And "We are under investigation"
I have repeatedly said there IS an investigation. Just that Cytodyn has not stated that they are the ones under investigation.
Would Cytodyn disclose that there is an investigation in their Annual Report if Amazon was the company being investigated?
Quote: Mission146Would Cytodyn disclose that there is an investigation in their Annual Report if Amazon was the company being investigated?
If they had received subpeona's about it yes.
Quote: darkozIf they had received subpeona's about it yes.
I'll put it this way: My parody I did a few pages back was not-so-loosely based on what actually happened. They obviously didn't come out and say that LemonLamb cures the Coronavirus, but they stated, essentially, that they were testing it and thought it had promise with absolutely no reason to actually believe that.
"Actually, Bill, you need to actually read articles instead of relying on headlines.
Please link to anywhere the company said they were under investigation.
Let me tell you that you can't. Sorry, the headline is lying and in fact in the article they cite the actual things Cytodyn said (hint, they never said they were under investigation by anyone)."
From CyDys annual report:
The Company is cooperating fully with these non-public, fact-finding investigations, and as of the date of this filing, the Company is unable to predict the ultimate outcome and cannot reasonably estimate the potential possible loss or range of loss, if any.
Quote: darkozIf they had received subpeona's about it yes.
That's not how things work. CYDY reported the investigation because they have a fiduciary duty to do so and their outside auditors would not sign off on the Annual Report without the disclosures. When a company puts out press releases, a certain amount of puffery is allowed. Annual reports are a financial document and the good and the bad must be reported.
Quote: Mission146I'll put it this way: My parody I did a few pages back was not-so-loosely based on what actually happened. They obviously didn't come out and say that LemonLamb cures the Coronavirus, but they stated, essentially, that they were testing it and thought it had promise with absolutely no reason to actually believe that.
Why would you say they had absolutely no reason to believe it works for coronavirus?
The trial was FDA approved.
Are you saying the FDA had no reason to believe it either?
That's literally what any such investigation would have to prove
Not only did Cytodyn conduct trials for Covid that failed but they knew it would fail and so did the FDA.
If of course the FDA didn't know it would fail and went forward with the trials..
Quote: DRichDarkoz, why are you so passionate about CYDY? It is just a small company that will either be successful or not. Investments work out sometimes and sometimes they don't. Que sera sera
I'm passionate because I believe in the science behind it and it can save many lives.
It's been in HIV testing for over seven years.
And yes, I am monetarily invested as well.
The real question is why is Bill Ryan so passionate about disproving Leronlimab?
It's almost feels like he is shorting the stock.
Que Sera Sera.
Quote: darkozWhy would you say they had absolutely no reason to believe it works for coronavirus?
The trial was FDA approved.
Are you saying the FDA had no reason to believe it either?
That's literally what any such investigation would have to prove
Not only did Cytodyn conduct trials for Covid that failed but they knew it would fail and so did the FDA.
If of course the FDA didn't know it would fail and went forward with the trials..
Yeah, the trial WAS FDA approved, you have no reason to believe that something actually works or doesn't work until the trial has been completed---that's why you have clinical trials. They had no more reason to believe that LemonLamb works on Coronavirus than they hypothetically would to believe that it works on Down Syndrome.
Given the pandemic nature of Covid-19, I would think that the FDA wouldn't be shy about approving trials for something to deal with it. As you can see, we got the vaccines with a much faster turnaround than is typical.
LemonLamb trials were already going on for HIV treatment as well as Triple-Negative breast cancer prior to Covid-19 becoming a thing. For those reasons, I assume (and could be wrong) it had become pretty much accepted that LemonLamb is not going to harm a person. Given that it's not actually harmful, why not run it through a trial and see if it yields positive results?
More than that, the FDA was also angry that Cytodyn, in their opinion, misrepresented the results of the trial:
https://www.biospace.com/article/fda-torpedoes-cytodyn-s-leronlimab-for-covid-19/
That article links to the FDA's statement:
https://www.fda.gov/drugs/drug-safety-and-availability/statement-leronlimab
The second link basically says that the trials found that LemonLamb did nothing or almost nothing. Any improvements v. a placebo, what few could be found, were statistically insignificant.
Because Cytodyn is a publicly-traded pharmaceutical research company testing exactly one drug, any public statements made that would result in a scolding from the FDA are of interest to the SEC because they could result in people wanting to invest in Cytodyn based, not only on misleading information, but on misleading information that the company itself was responsible for putting out there.
That's not to say that the SEC's investigation will come to any such conclusion. I think we can all agree that we don't know yet what the conclusion will be.
Quote: darkozI'm passionate because I believe in the science behind it and it can save many lives.
It's been in HIV testing for over seven years.
And yes, I am monetarily invested as well.
The real question is why is Bill Ryan so passionate about disproving Leronlimab?
It's almost feels like he is shorting the stock.
Que Sera Sera.
I still don't understand the passion. It will either work or it won't. Revisit it in 10 years and you can say you were right or wrong. I think it is ridiculous that both of you go back and forth. Why not just wait and see who is right.
https://www.cytodyn.com/newsroom/press-releases/detail/551/cytodyns-final-mtnbc-report-indicates-as-much-as-980
I think we can all agree that there is an SEC investigation, which was all I was saying from the get go. I wasn't aware of the DOJ investigation and if oz hadn't insisted the company never said what is said, I would not have found out about it. For that, I suppose, we should thank him.
Quote: Mission146In the midst of all of this, they have released some promising looking data on the cancer front:
https://www.cytodyn.com/newsroom/press-releases/detail/551/cytodyns-final-mtnbc-report-indicates-as-much-as-980
I linked to that yesterday and Bill claims it's a propaganda piece, lol.
Quote: Mission146In the midst of all of this, they have released some promising looking data on the cancer front:
https://www.cytodyn.com/newsroom/press-releases/detail/551/cytodyns-final-mtnbc-report-indicates-as-much-as-980
Keep in mind that their promising looking data from the Spring turned out to be complete bullspit and was what got the FDA and SEC involved in the first place, so I'd take this press release with more than a couple grains of salt.
Current Rating
Strong Sell
Previous Rating
Strong Sell
Quote: billryanKeep in mind that their promising looking data from the Spring turned out to be complete bullspit and was what got the FDA and SEC involved in the first place, so I'd take this press release with more than a couple grains of salt.
I have no position on Cytodyn and no intention to initiate any new positions within the next 72 hours, or ever, for that matter.
Quote: Mission146Yeah, the trial WAS FDA approved, you have no reason to believe that something actually works or doesn't work until the trial has been completed---that's why you have clinical trials. They had no more reason to believe that LemonLamb works on Coronavirus than they hypothetically would to believe that it works on Down Syndrome.
Given the pandemic nature of Covid-19, I would think that the FDA wouldn't be shy about approving trials for something to deal with it. As you can see, we got the vaccines with a much faster turnaround than is typical.
LemonLamb trials were already going on for HIV treatment as well as Triple-Negative breast cancer prior to Covid-19 becoming a thing. For those reasons, I assume (and could be wrong) it had become pretty much accepted that LemonLamb is not going to harm a person. Given that it's not actually harmful, why not run it through a trial and see if it yields positive results?
More than that, the FDA was also angry that Cytodyn, in their opinion, misrepresented the results of the trial:
https://www.biospace.com/article/fda-torpedoes-cytodyn-s-leronlimab-for-covid-19/
That article links to the FDA's statement:
https://www.fda.gov/drugs/drug-safety-and-availability/statement-leronlimab
The second link basically says that the trials found that LemonLamb did nothing or almost nothing. Any improvements v. a placebo, what few could be found, were statistically insignificant.
Because Cytodyn is a publicly-traded pharmaceutical research company testing exactly one drug, any public statements made that would result in a scolding from the FDA are of interest to the SEC because they could result in people wanting to invest in Cytodyn based, not only on misleading information, but on misleading information that the company itself was responsible for putting out there.
That's not to say that the SEC's investigation will come to any such conclusion. I think we can all agree that we don't know yet what the conclusion will be.
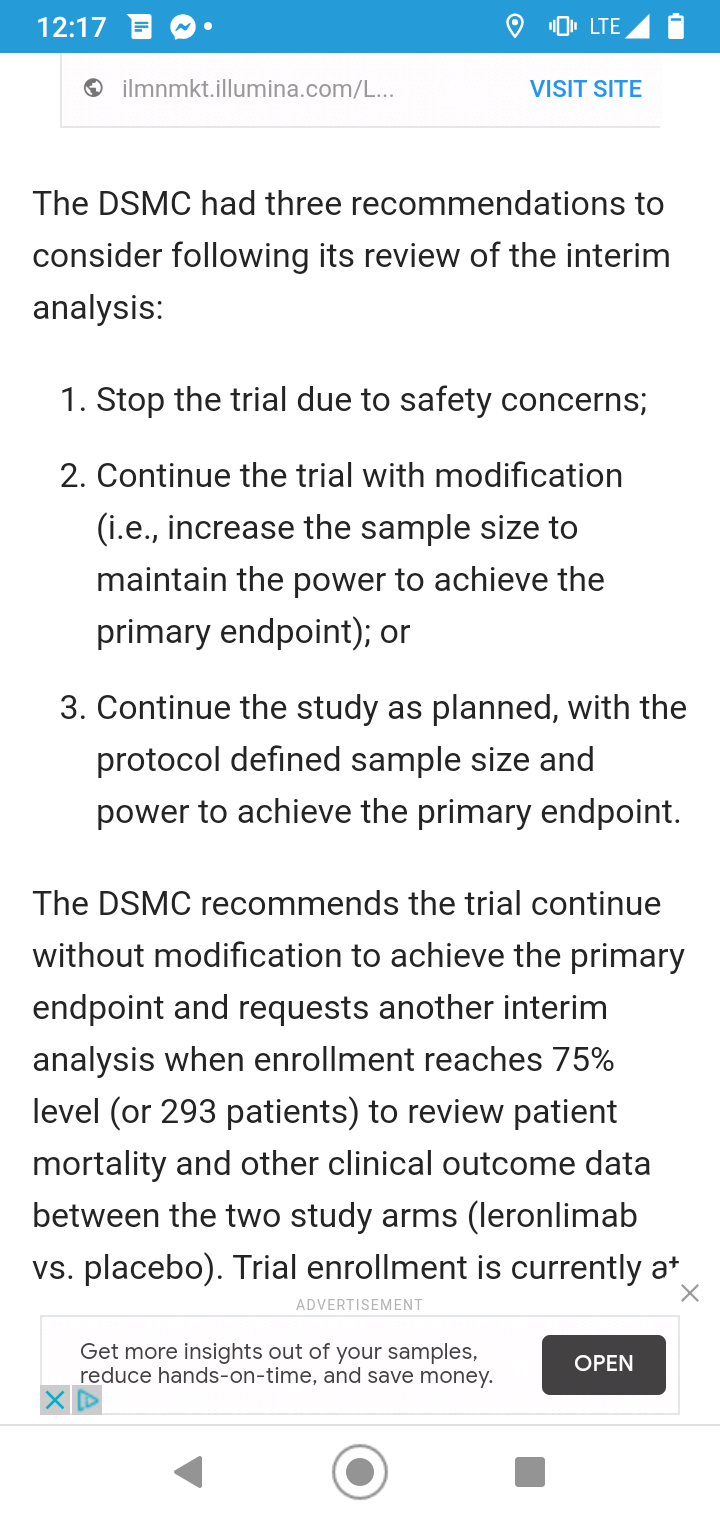
Mission,
The trials are not just approved by the FDA.
They are monitored by the DSMC.
The DSMC is an independent board that takes interim looks at drugs to determine if they are safe and efficacious.
For example, if the drug didn't work at all, the DSMC would shut the trial down. No use having patients drugged and still dying nor wasting hospital resources.
The DSMC gave Leronlimab the green light.
So not only the FDA and Cytodyn but the independent DSMC (the only organization that had a view of the double-blind data) felt Leronlimab was a possibility for working on covid.
https://www.drugs.com/clinical_trials/cytodyn-receives-positive-dsmc-recommendation-after-interim-analysis-leronlimab-phase-2b-3-covid-19-19006.html
The FDA publicly scolded Cytodyn for what they said about LemonLamb and the Covid-19 trial in their press releases. I do not know what specific press releases are being referred to and don't consider this matter important enough for me to look into it. I'm simply maintaining that the company was publicly scolded for whatever it represented in its press release(s) after the first trial was conducted. That's an immutable fact for which I have provided evidence.
I don't have a problem with Cytodyn in particular; I don't even know why I have been reading this thread. However, if I am going to read it, then I am going to point out facts (as long as they are readily available) that would contradict something to the contrary of facts being stated in a post---regardless of who posts it.
The thing that you quoted recommended that they continue the trial. I would assume that the FDA is not upset that they recommended that and Cytodyn parroted that recommendation, but I could be wrong.
What an awful crime when those in the leronlimab covid trial were denied dosing after second dose.... and then passed. The trial was a set up to fail and should have never been agreed to by the company. It was like watching a prize fight in Vegas with one of the contenders hand tied behind his back. A system that is compassion limited and that works in opposition to the Hippocratic Oath must be replaced. We have no other choice if we want to advance as a culture. That public scolding of CytoDyn by the FDA was highly unusual. Deviously and intentionally a political hit job written in a way to mislead the public. A passing trial and approval in Brazil will be a real smack down to the USA FDA. They deserve a knock out punch.
Quote: AuraAngel2tuFDA has been fraught with corruption going way back and has only gotten worse with time passing. DSMC has had a few snafus as well. As someone said, it either works or it doesn't. Yes, I agree. Too bad the FDA doesn't work like that. /2021/06/11/1005567149/3-experts-have-resigned-from-an-fda-committee-over-alzheimers-drug-approval
What an awful crime when those in the leronlimab covid trial were denied dosing after second dose.... and then passed. The trial was a set up to fail and should have never been agreed to by the company. It was like watching a prize fight in Vegas with one of the contenders hand tied behind his back. A system that is compassion limited and that works in opposition to the Hippocratic Oath must be replaced. We have no other choice if we want to advance as a culture. That public scolding of CytoDyn by the FDA was highly unusual. Deviously and intentionally a political hit job written in a way to mislead the public. A passing trial and approval in Brazil will be a real smack down to the USA FDA. They deserve a knock out punch.
Since we’re talking about the stock and you’re new, may I ask if you currently have a position or intend to initiate one? As far as I know, you’re not legally required to answer that question if you do not wish to do so.
Quote: Mission146Since we’re talking about the stock and you’re new, may I ask if you currently have a position or intend to initiate one? As far as I know, you’re not legally required to answer that question if you do not wish to do so.
He probably does.
But he brought up something that I felt would get pooh-poohed on here so let me go into detail.
The FDA insisted on the trial having only two doses AND examination of results 28 days later.
The drug has a 7 day response and has been used this manner for HIV for years. That is HIv patients get one shot every week.
HIV ocf course is a lifetime disease. Covid-19 isn't so you wouldn't need a lifetime of weekly injections. Just four if you are going to evaluate it at 28 days.
The FDA insisted on two doses only.
Dose at day zero
Dose at day seven (patients examined showed 79% better than placebo)
Day fourteen (no third dose but seven days after last dose patients showed 84% better results than placebo)
Day twenty-one (two weeks since last dose patients showed 54% better than placebo)
Day twenty-eight (three weeks since last dose patients showed only 24% better than placebo which made them not qualify for their P-value (P-value is a measurement score for drug efficacy)
So basically the drug efficacy got worse the further out from being dosed.
This is equivalent to testing out a seven day antibiotic and insisting the trial only go for three days dosage, then complaining the drug failed to work.
The trial should have been allowed to go four doses. The new trials in Brazil that are starting are four doses proper.
I mean, what a novel idea, actually administering the drug properly to see if it works.
All of this get into conspiracy territory (hence my reluctance to bring it up) but it does seem FDA has been compromised when (as the new poster linked to) another drug that all independent agencies said doesn't work got FDA approval for this big pharmaceutical company leading to resignations.
The conspiracy theory is that big pharma companies don't want any drug that will endanger their cash cows, like a drug that beats cancer, covid and HIV. So they convince the FDA to slow walk or sabotage the trials.
BTW, FDA is financed by big pharma. Like, literally they get more than half their funding from big pharma companies. No conflicts of interest there, right?
Had the FDA really cared, they would have allowed at least 4 doses, one each week. You take a poor fella about dead and give him just a little medicine and expect recovery. Amazing thing is some did recover at a higher rate than SOC and placebo. Yes, I am very aware of FDA with their hands out taking BP money. This should not be allowed. It is like lobbyist being able to pay congressmen for their votes. A couple of my friends sell pharma drugs for a living. They know how to bribe and schmooze the hospitals and doctors. Everyone likes perks. Vegas thrives off of it. Remember the junkets? Now those were the days!
Quote: Mission146<snip>
I don't have a problem with Cytodyn in particular; I don't even know why I have been reading this thread. <snip>
I've read this entire thread and found this to be the funniest line so far. Carry on.
Quote: darkozDRich linked to an article about Cytodyn receiving the patent on Leronlimab. He wasn't suggesting investing on the way down on a hope but on information that the company has hit a legal milestone.
If Leronlimab's molecule is synthesized by any other pharmaceutical company they will owe patent fees or whatever.
Leronlimab has shown extremely good results. It's missed it's end points by a very small margin which is why the FDA refused to okay it (even though a few competing drugs that failed the mark by worse got approved thanks to their big pharma connection but hey welcome to corruption).
Point is this close you don't say "Ok, we were close, next!". What you do is figure out what is needed to hit the mark. Different dosage, different schedule of application etc.
And now if some big pharma company tries to step in and develop their own version guess what, they get a letter of patent from Cytodyn.
So based on information, not "gee, otc sucks" DRich is asking isn't this a good time to buy.
Dark oz keeps insisting lemonlabob missed its end points by a small margin while the FDA says it was a total failure and produced results similar to the placebo.
Who should we believe?
Quote: billryanDark oz keeps insisting lemonlabob missed its end points by a small margin while the FDA says it was a total failure and produced results similar to the placebo.
Who should we believe?
I don't know what lemonlabob is. Sorry if you invested in that.
As for Leronlimab the FDA said they are prepared to continue with trials.
Not exactly the words "Total failure" which you claim they said (nowhere in the FDA statement are the words Total failure used. That's Bill Ryans words. Who's words are you going to believe).
Here is the FDA final statement on the subject. Mentioning forward trials. Not exactly what one would expect of a "total failure".

So apparently Bill believes that when a drug is a "total failure" the FDA discusses and continues trials.
LMFAO.
With the conclusion of both the CD10 and CD12 clinical trials, it has become clear that the data currently available do not support the clinical benefit of leronlimab for the treatment of COVID-19.
In the smaller study that CytoDyn conducted in patients with mild-to-moderate COVID-19 disease (CD10), there was no observed effect of the drug on the study’s primary endpoint or on any of the secondary endpoints
The primary endpoint for the CD10 trial relied on a measure of participants’ COVID-19 symptoms called a “total clinical symptom score”, which was assigned based on the severity of each participant’s fever, muscle aches, shortness of breath, and cough. This score ranged from 0 (no symptoms) to 12 (all 4 symptoms present and severe). The CD10 trial results showed no clinically meaningful differences in average change in “total clinical symptom score” from baseline to Day 14 between study arms (-3.5 in the leronlimab group versus -3.4 in the placebo group). Additionally, none of the secondary endpoints were met in this study, including mortality, time to symptom resolution, and time to return to normal activity. Taken together, the CD10 results indicate that most study participants experienced resolution in COVID-19 symptoms regardless of whether they received leronlimab or placebo.
The larger trial that CytoDyn conducted in patients with severe COVID-19 disease (CD12) also failed to find any effect of the drug on the primary study endpoint, with no difference seen in mortality (20.5% in the leronlimab treatment group and 21.6% in the placebo treatment group); or on any of the secondary endpoints, for example, with no difference on the average length of hospitalization (21.4 days in both the leronlimab and the placebo treatment groups).
CytoDyn has publicly communicated differences in small subgroups from the CD12 trial (e.g., a sub-group analysis of 62 of the 394 patients studied) suggesting that the data demonstrated a mortality benefit in certain patients who had received leronlimab. Subgroup analyses have well-established limitations, especially in the context of a clinical trial that has failed to show a benefit in the overall study population. For example, subgroups are often small, and therefore imbalances are common. Here, the data from CD12 illustrated imbalances in mortality among subgroups, some favoring leronlimab and some favoring placebo. None of these analyses met statistical significance when using established and reliable analytical methods that correct for multiple comparisons. However, as noted above, such analyses may inform the design of future clinical trials investigating leronlimab for the treatment of COVID-19.
Failed to find any effect....
no difference on the average length.....
no observed effects.....
I have no idea why oz continues to post things that are verifiably untrue. I can't judge his motives but it is clear he refuses to recognize the reality of these trials.
I'll repeat. The FDA says the CD10 and CD12 trials did not support any benefit for the treatment of Covid 19. It's all there in the very document oz selectively edited to attempt to prove his point.
Quote: billryanDark oz quotes the last piece of the document but leaves out this:
With the conclusion of both the CD10 and CD12 clinical trials, it has become clear that the data currently available do not support the clinical benefit of leronlimab for the treatment of COVID-19.
In the smaller study that CytoDyn conducted in patients with mild-to-moderate COVID-19 disease (CD10), there was no observed effect of the drug on the study’s primary endpoint or on any of the secondary endpoints
The primary endpoint for the CD10 trial relied on a measure of participants’ COVID-19 symptoms called a “total clinical symptom score”, which was assigned based on the severity of each participant’s fever, muscle aches, shortness of breath, and cough. This score ranged from 0 (no symptoms) to 12 (all 4 symptoms present and severe). The CD10 trial results showed no clinically meaningful differences in average change in “total clinical symptom score” from baseline to Day 14 between study arms (-3.5 in the leronlimab group versus -3.4 in the placebo group). Additionally, none of the secondary endpoints were met in this study, including mortality, time to symptom resolution, and time to return to normal activity. Taken together, the CD10 results indicate that most study participants experienced resolution in COVID-19 symptoms regardless of whether they received leronlimab or placebo.
The larger trial that CytoDyn conducted in patients with severe COVID-19 disease (CD12) also failed to find any effect of the drug on the primary study endpoint, with no difference seen in mortality (20.5% in the leronlimab treatment group and 21.6% in the placebo treatment group); or on any of the secondary endpoints, for example, with no difference on the average length of hospitalization (21.4 days in both the leronlimab and the placebo treatment groups).
CytoDyn has publicly communicated differences in small subgroups from the CD12 trial (e.g., a sub-group analysis of 62 of the 394 patients studied) suggesting that the data demonstrated a mortality benefit in certain patients who had received leronlimab. Subgroup analyses have well-established limitations, especially in the context of a clinical trial that has failed to show a benefit in the overall study population. For example, subgroups are often small, and therefore imbalances are common. Here, the data from CD12 illustrated imbalances in mortality among subgroups, some favoring leronlimab and some favoring placebo. None of these analyses met statistical significance when using established and reliable analytical methods that correct for multiple comparisons. However, as noted above, such analyses may inform the design of future clinical trials investigating leronlimab for the treatment of COVID-19.
Failed to find any effect....
no difference on the average length.....
no observed effects.....
I have no idea why oz continues to post things that are verifiably untrue. I can't judge his motives but it is clear he refuses to recognize the reality of these trials.
I'll repeat. The FDA says the CD10 and CD12 trials did not support any benefit for the treatment of Covid 19. It's all there in the very document oz selectively edited to attempt to prove his point.
Notice Bill left out pointing to his quote "total failure"
Because nowhere does it say that
Nowhere in this world does a drug have total failure and then the FDA says they are fine with moving forward on similar trials of the same drug.
Bill doesn't understand how things work even though he tries very hard.
I have no idea why Bill is so anxious to prove Leronlimab failure. He has nothing invested in this apparently except some form of bravado by saying"I told you so".
So he leaves out and discounts what doesn't suit his agenda.
no difference on the average length.....
no observed effects.....
Perhaps dark oz looks at those phrases and reads " success".
I notice the FDA says "Failed to find any effect. " Perhaps they really meant It succeeded to not find any effect, or that they successfully showed no difference on the average length.
Thats the ticket. They didn't fail to find any observed effects between the drug and the placebos. They successfully proved lemonlabob was equally effective as the placebos.
Quote: billryanFailed to find any effect....
no difference on the average length.....
no observed effects.....
Perhaps dark oz looks at those phrases and reads " success".
I notice the FDA says "Failed to find any effect. " Perhaps they really meant It succeeded to not find any effect, or that they successfully showed no difference on the average length.
Thats the ticket. They didn't fail to find any observed effects between the drug and the placebos. They successfully proved lemonlabob was equally effective as the placebos.
I'm sorry you don't fully comprehend the documents from the FDA or lack understanding of clinical trials.
When the drug is a total failure trials do not move forward.
However when trials show they didn't have the intended effect but there appears from the science a way forward, then new trials are designed.
These trials will be designed to see if the dosage can be improved, time, outcomes, etc.
The FDA pretty much says as such when they conclude there is a path forward for future trials.
Quoting three words out of the document to make your case is sad. I understand you think that those three words prove your case but again the FDA conclusion (and after all the conclusion remarks are what is most important) is that Cytodyn can use the previous trials as exploratory for further trials.
That simply isn't the medical definition of total failure for a biotech drug.
You can keep quoting a few words here and there but the final conclusion by the FDA approving a way forward doesn't change.
Quote: billryanYou are the one who selectively quoted from the document. I posted the part you left out.
I quoted the part of the document that refuted your claims that the FDA cited "total failure".
The relevant part to refute that was the part I quoted.
Interestingly, by posting the rest of the document, you refute yourself. Nowhere are the words "total failure" used.
You made that up.
Again, it's clear it isn't total failure as the FDA says they will be fine moving forward with trials that can use the previous trials as exploratory.
The larger trial that CytoDyn conducted in patients with severe COVID-19 disease (CD12) also failed to find any effect of the drug on the primary study endpoint, with no difference seen in mortality (20.5% in the leronlimab treatment group and 21.6% in the placebo treatment group); or on any of the secondary endpoints.
What they really mean is lemonlabob came so close.
Your argument seems to be that the FDA announcing the drug failed every aspect of the trials, didn't achieve any of the studies endpoints or any of the secondary endpoints doesn't equal failure. Meanwhile you keep claiming it just missed it's marks. I'm not sure how you think of success, but I would think a rationale person would look at a project that failed to achieve even one objective as a failure. The FDA said they are welcome to try again. Is that your standard of success?
I wonder if shareholders considers his investment a total failure.
Quote: billryanI suppose when the FDA says this-
The larger trial that CytoDyn conducted in patients with severe COVID-19 disease (CD12) also failed to find any effect of the drug on the primary study endpoint, with no difference seen in mortality (20.5% in the leronlimab treatment group and 21.6% in the placebo treatment group); or on any of the secondary endpoints.
What they really mean is lemonlabob came so close.
Your argument seems to be that the FDA announcing the drug failed every aspect of the trials, didn't achieve any of the studies endpoints or any of the secondary endpoints doesn't equal failure. Meanwhile you keep claiming it just missed it's marks. I'm not sure how you think of success, but I would think a rationale person would look at a project that failed to achieve even one objective as a failure. The FDA said they are welcome to try again. Is that your standard of success?
I wonder if shareholders considers his investment a total failure.
You now need to change the goalposts.
I never claimed the trials were a success. I pointed to the need for additional trials as did the FDA!
You claimed the FDA "said total failure". Yet nowhere did the FDA say those words "total failure".
So now changing the goalposts is your only way to save face.
The real problem with your argument is that it discounts the moving forward with trials. Let's say a new set of trials meets endpoints next year.
Will that classify as "total failure"?
Of course not, that's ridiculous.
If you said the FDA claimed the drug failed current trials I would give you that. But you always interpret things to the extreme so suddenly you are quoting something not said (total failure).
FDA:....... failed to find any effect of the drug on the primary study endpoint, with no difference seen in mortality (20.5% in the leronlimab treatment group and 21.6% in the placebo treatment group); or on any of the secondary endpoints.
Oz claims extremely good results vs the FDA saying it failed to find any effect, or on any secondary endpoints.
As far as you saying you never claimed the trial was a success, do you really want me to posts your comments from when the trials results were first made public? I'm tracking two auctions right now and have to be on the web anyways.
Quote: billryan... the FDA says it was a total failure...
Looking for those words Bill.
Please point to those words or you made it up?
(Notice how SOOPOO is not really involved in the bet….)
Quote: SOOPOOCYDY is the best performing stock in the WoV portfolio today, up 3.5%. 5 more days like this and I’m back to even! It’s $1.33. I’m proposing a bet…. Pick some Wizard approved charity…. If CYDY drops below $1 DarkOz makes the donation. If it eclipses $1.66 Billy fills the charity’s coffers. First event to occur, wins.
(Notice how SOOPOO is not really involved in the bet….)
I've already made my wager
It's on Cytodyn stock.
An oldie but a goodie:
August 14th, 2020 at 9:29:12 PMpermalink
This company was dead in the water, with its stock being worth a nickel when a man with a doctorate in Mechanical Drawing and no pharma experience was able to wrangle control of it. He convinced a few private investors to pony up 12 million dollars and replaced the board of directors that was heavily pharma oriented with people associated with the private investors. They then announced plans to research, develop, and market a drug that had an estimated annual market in the 20 million dollar range. Because of its limited market, they were able to fast track it under the FDAs "Orphan drug" program where they claim a drug has limited potential and isn't worth the money to go through the usual process. Then they tried to tie this drug in with every disease under the sun, and proclaimed it had a potential cap of between 2.5 billion and 9 billion, despite not having produced a single dose for commercial use. In line with their 9 billion dollar potential cap, they make a splashy deal with the infamous Martin Shkreli's Turing Pharma under its newest name. The deal sounds great, $8.75 billion dollar contract, only it's worthless until the FDA approves the drug for that particular use.
Suddenly Corona virus hits, the company makes some incredulous claims without any evidence, the stock soars and insiders start dumping shares.
Quote: billryanOz says he paid $4 a share.
An oldie but a goodie:
August 14th, 2020 at 9:29:12 PMpermalink
This company was dead in the water, with its stock being worth a nickel when a man with a doctorate in Mechanical Drawing and no pharma experience was able to wrangle control of it. He convinced a few private investors to pony up 12 million dollars and replaced the board of directors that was heavily pharma oriented with people associated with the private investors. They then announced plans to research, develop, and market a drug that had an estimated annual market in the 20 million dollar range. Because of its limited market, they were able to fast track it under the FDAs "Orphan drug" program where they claim a drug has limited potential and isn't worth the money to go through the usual process. Then they tried to tie this drug in with every disease under the sun, and proclaimed it had a potential cap of between 2.5 billion and 9 billion, despite not having produced a single dose for commercial use. In line with their 9 billion dollar potential cap, they make a splashy deal with the infamous Martin Shkreli's Turing Pharma under its newest name. The deal sounds great, $8.75 billion dollar contract, only it's worthless until the FDA approves the drug for that particular use.
Suddenly Corona virus hits, the company makes some incredulous claims without any evidence, the stock soars and insiders start dumping shares.
$3.44 per share at this time.
Where is the link or credit for the quote?
And what do bloggers or opinions from whoever posted this matter?
Trials are ongoing. The FDA looking forward to further trials. If you feel the drug is such an unmitigated disaster why don't you write the FDA asking why they are willing to move forward with future trials for a drug that you believe is worthless?
Quote: darkozLooking for those words Bill.
Please point to those words or you made it up?
Is the whole point of your inane argument that the FDA only said the trial failed to reach achieve any of it's endpoints or its secondary points but didn't use the words total failure.
Meanwhile, you keep insisting it almost met its mark.
Quote: darkoz$3.44 per share at this time.
Where is the link or credit for the quote?
And what do bloggers or opinions from whoever posted this matter?
Trials are ongoing. The FDA looking forward to further trials. If you feel the drug is such an unmitigated disaster why don't you write the FDA asking why they are willing to move forward with future trials for a drug that you believe is worthless?
$1.31 in the real world. Evidently $3.44 in Oz's.
Huh? He’s saying his weighted average buy price is $3.44 at this time.Quote: billryan$1.31 in the real world. Evidently $3.44 in Oz's.
Quote: billryanIs the whole point of your inane argument that the FDA only said the trial failed to reach achieve any of it's endpoints or its secondary points but didn't use the words total failure.
Meanwhile, you keep insisting it almost met its mark.
Because you don't understand medical trials.
Have you examined all the data? Not the single snippet supplied by the FDA which is hardly the total picture but all the data.
Missing the endpoints are a matter of P-value measurements with P-value needing to be less than .005
So if the results are .006, that's failure.
I would say that's just missing the mark but missed nonetheless.
That type of miss (and I don't have the numbers up in front of me but they were close) is why the FDA didn't say your ridiculous words "Total failure".
That's why the FDA is looking forward to future trials if the company decided to move forward.
I understand your lack of knowledge in this area makes you just see the words"failed endpoints" as more than the total story.
You claimed you have done very little research into the science. This is the result.
Quote: unJonHuh? He’s saying his weighted average buy price is $3.44 at this time.
Thank you, Unjon.
Precisely.
Say what? Now I'll play your elementary school games. Where did the FDA say they were looking forward to another trial by them?
This has nothing to do with DarkOz, but I wanted to take a moment and just say for anyone reading that blind, "Average Down," is a terrible strategy, unless you have a good reason to be doing it. I couldn't even tell you how many individual stocks have resulted in people getting killed on that, and that's just of people I have talked to.
Remember this---if you're averaging down, then the stock is already worth less (market price) than it was when you bought it.
Instead, don't average down for its own sake. This is especially true if you are an investor, as opposed to a trader. Ask yourself, "Based on the information I have now, which is more information than I had when I first bought the stock, would I buy the stock at this price if I did not already own it?"
If the answer is yes, then you might consider picking up more.
If the answer is no, then do not average down.
This can sometimes be different for traders who might be expecting (for one reason or another) a short-term bump up in price, but not quite to the price point where they made their initial stock buy.
NOTE: It sounds like Darkoz believes in this company, so he might have been averaging down because he saw the lower share price as a bargain and actually expects the price to eventually climb above his initial buy point.
https://wizardofvegas.com/articles/playing-the-pennies-stocks/
Please keep in mind that I am not an expert, not even close. I have a passing knowledge of how everything works and a background in Economics that I mostly don't use for anything. That said, it takes a general look at things to look out for and a deep look into TOP Ships (TOPS) which is actually a Nasdaq listed company and, arguably, the most guilty company of any (that is actually listed still) when it comes to price manipulation games.
Quote: Mission146Average Down
This has nothing to do with DarkOz, but I wanted to take a moment and just say for anyone reading that blind, "Average Down," is a terrible strategy, unless you have a good reason to be doing it. I couldn't even tell you how many individual stocks have resulted in people getting killed on that, and that's just of people I have talked to.
Remember this---if you're averaging down, then the stock is already worth less (market price) than it was when you bought it.
Instead, don't average down for its own sake. This is especially true if you are an investor, as opposed to a trader. Ask yourself, "Based on the information I have now, which is more information than I had when I first bought the stock, would I buy the stock at this price if I did not already own it?"
If the answer is yes, then you might consider picking up more.
If the answer is no, then do not average down.
This can sometimes be different for traders who might be expecting (for one reason or another) a short-term bump up in price, but not quite to the price point where they made their initial stock buy.
NOTE: It sounds like Darkoz believes in this company, so he might have been averaging down because he saw the lower share price as a bargain and actually expects the price to eventually climb above his initial buy point.
I don't disagree with this at all, except if you are buying a certain amount per month. All through college, I bought
$20 a month of ATT and $15 a month from a company that eventually merged with Proctor and Gamble. In those cases, I wouldn't even look at the price, be it up or down. Back in those days, I had to actually write a check out and mail it to them. In general, buying on the down is a rookie mistake that almost everyone makes.


